Pupil response is governed by the autonomic nervous system. Pupil dilation depends on the activation of the adrenergic sympathetic nervous system, while pupil constriction depends on the cholinergic parasympathetic nervous system[1]. It has been well documented that pupil response is modulated not only by an ambient luminance level (the so-called pupil light reflex) but also by the amount of mental effort invested in a task [2][3][4][5][6][7][8][9][10][11][12]. For example, Porter et al[13] reported that the pupil dilates when subjects conduct a difficult visual search task in which high mental effort has to be invested.
.Converging evidence suggests that baseline pupil diameter is correlated with tonic levels of LC-NE activity in rats, cats and monkeys[14][15], as with human behaviors that are predicted to be associated with tonic LC-NE activity in a variety of experimental tasks and manipulations[16][17][18][19]
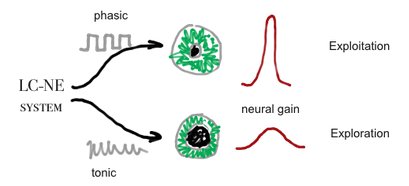
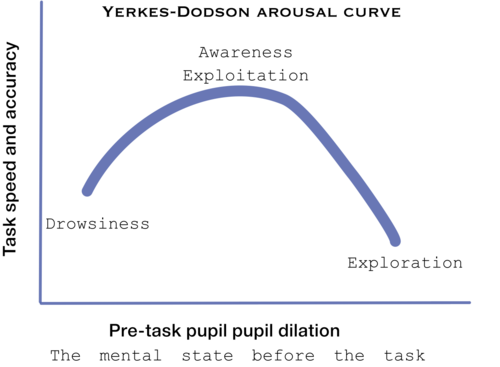
Although, no mechanism was found yet to the connection of pupil size and LC-NE system, Murphy et al (2011) found that p300 and pupil dilation is highly corrolated with LC-EN system behviour, according to the Yerkes-Dodson arousal curve[20]:
Pupil size is thought to be controled by the LC-NE system see Cohen[21]and Jepma & Nieuwenhuis[22]. Neural gain, may be the same as mind clouser
Oxitocin enhanced social detecting, and also pupil size (probably because of enhanced learning and attention)[23]
References
- ↑ Barbur J (2004) Learning from the pupil: studies of basic mechanisms and clinical applications. The Visual Neurosciences Vol. 1. In: Chlupa L, Werner J, editors. Cambridge: The MIT Press. pp. 641–656.
- ↑ Beatty J (1982) Task-evoked papillary responses, processing load, and the structure of processing resources. Psychological Bulletin 91: 276–292. doi: 10.1037/0033-2909.91.2.276
- ↑ Hampson RE, Opris I, Deadwyler SA (2010) Neural correlates of fast pupil dilation in nonhuman primates: relation to behavioral performance and cognitive workload. Behavioral Brain Research 212: 1–11.
- ↑ Kahneman D, Beatty J (1966) Pupil diameter and load on memory. Science 154: 1583–1585. doi: 10.1126/science.154.3756.1583
- ↑ Kahneman D, Peavler W (1969) Incentive effects and papillary changes in association learning. Journal of Experimental Psychology 79: 312–318.
- ↑ Partala T, Surakka V (2003) Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies 59: 185–198. doi: 10.1073/pnas.88.11.4966
- ↑ Porter G, Troscianko T, Gilchrist I (2007) Effort during visual search and counting: insights from pupilometry. The Quarterly Journal of Experimental Psychology 60: 211–229. doi: 10.1080/17470210600673818
- ↑ van Orden KF, Jung T-P, Makeig S (2000) Combined eye activity measures accurately estimate changes in sustained visual task. Biological Psychology 52: 221–240. doi: 10.1016/S0301-0511(99)00043-5
- ↑ van Orden KF, Limbert W, Makeig S, Jung T-P (2001) Eye activity correlates of workload during visuospatial memory task. Human Factors 43: 111–121. doi: 10.1518/001872001775992570
- ↑ Heaver, Becky, and Sam B. Hutton. "Keeping an eye on the truth? Pupil size changes associated with recognition memory." Memory 19.4 (2011): 398-405.
- ↑ Silvetti, Massimo, et al. "The influence of the noradrenergic system on optimal control of neural plasticity." Frontiers in behavioral neuroscience 7 (2013).
- ↑ Takeuchi, Tatsuto, et al. "Estimation of Mental Effort in Learning Visual Search by Measuring Pupil Response." PloS one 6.7 (2011): e21973.
- ↑ Porter G, Troscianko T, Gilchrist I (2007) Effort during visual search and counting: insights from pupilometry. The Quarterly Journal of Experimental Psychology 60: 211–229. doi: 10.1080/17470210600673818
- ↑ Aston-Jones, G. & Cohen, J.D. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
- ↑ Koss, M.C. Pupillary dilation as an index of central nervous system α2-adrenoceptor activation. J. Pharmacol. Methods 15, 1–19 (1986).
- ↑ Gilzenrat, M.S., Nieuwenhuis, S., Jepma, M. & Cohen, J.D. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn. Affect. Behav. Neurosci. 10, 252–269 (2010).
- ↑ Jepma, M. & Nieuwenhuis, S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. J. Cogn. Neurosci. 23, 1587–1596 (2011).
- ↑ Einhäuser, W., Stout, J., Koch, C. & Carter, O.L. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. USA 105, 1704–1709 (2008).
- ↑ Murphy, P.R., Robertson, I.H., Balsters, J.H. & O'Connell, R.G. Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology 48, 1532–1543 (2011).
- ↑ Murphy, P. R., Robertson, I. H., Balsters, J. H., & O’connell, R. G. (2011). Pupillometry and P3 index the locus coeruleus--noradrenergic arousal function in humans. Psychophysiology, 48(11), 1532–1543.
- ↑ Eldar, Eran, Jonathan D Cohen & Yael Niv. 2013. The effects of neural gain on attention and learning. Nature Neuroscience. Published online June 16, 2013 doi:10.1038/nn.3428 (summery)
- ↑ Marieke Jepma and Sander Nieuwenhuis, 2011, Pupil Diameter Predicts Changes in thev Exploration – Exploitation Trade-off: Evidence for the Adaptive Gain Theory, Jurnal of Cognitive Neuroscience 23:7, pp. 1587-1596
- ↑ Siri Leknes, Johan Wessberg, Dan-Mikael Ellingsen, Olga Chelnokova1, Håkan Olausson and Bruno Laeng (2012) Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions